42 electrolysis of water lab answers
200 questions with answers in ELECTROLYSIS | Scientific method To produce one kg of H2 you need 9 kg of water. To get one molecule of hydrogen you need two electronic charges 2q. The charge Q required to get one kilograms= 2qx no. of moles in one kilograms ... Electrolysis of Water Lab - Electrolysis of Water Lab Purpose ... Electrolysis is the process of splitting water into Hydrogen and Oxygen by passing electricity through it. 5. Draw a model of a water molecule. Conclusion: The process used to separate water is called electrolysis. Electrolysis is when electricity is passed through water (H 2 O) and it splits into 2H2 and O 2 .
PDF Electrical Conductivity of Solutions answers Water is a poor conductor of electrical current which is why sulfuric acid was added during the electrolysis experiment. Solutions that conduct electrical current do so because ... Microsoft Word - Electrical Conductivity of Solutions_answers.doc Author: ptiskus

Electrolysis of water lab answers
Electrolysis of Water (A teacher demonstration experiment ... Lab # 1 Background Electrolysis of water is the process by which water is decomposed into oxygen and hydrogen gas, when electric current is passed through it. Water molecule is decomposed in to H+ and OH- ions, when electric current is passed through it. ELECTROLYSIS LAB WORKSHEETS Calculations The number ... ELECTROLYSIS LAB WORKSHEETS Calculations The number of moles of copper oxidized is obtained from the mass of copper oxidized. The number of moles of H2 collected is calculated from the ideal gas law, PV = nRT. The pressure of the hydrogen is not equal to the atmospheric pressure, and therefore two corrections must be made. Frequently Asked Questions | The Perfect Water Alkaline Water Ionizers Most of the popular alkaline water ionizers sold today use a separation technology known as electrolysis to separate the water into acidic and alkaline water streams. Electrolysis does not filter or purify the water, it simply divides the incoming water flow into an alkaline stream and an acidic stream. The secret that alkaline water treatment devices which …
Electrolysis of water lab answers. Electrochemistry: Electrolysis of Water Lab Electrolysis Solution X Post-Lab Questions: 1. Suggest an explanation for the initial indicator color of the electrolysis solution. 2. Describe at least three observations that indicate a chemical reaction has occurred during the electrolysis of water. 3. What are the two functions of the pencil-lead-electrodes? 4. experiment-electrolysis-of-water.pdf - Electrolysis of ... Electrolysis of Water (Chem 11 Lab Experiment) I. Introduction Electrolysis is the process in which electrical energy is used to cause a nonspontaneous chemical reaction to occur. An electrolytic cell is an apparatus for carrying out electrolysis. Electrolysis is often used to obtain elements that are too chemically reactive to be found free in nature. ... Electrolysis of Water Experiment - Splitting Water | HST Electrolysis is the process by which an electric current is passed through a substance to affect a chemical change. The chemical change occurs when the substance loses electrons (oxidation) or gains them (reduction). In the two experiments listed below, the first reactive substance is water and the second one is a copper sulfate solution. Electrolysis of Water - YouTube Experiment with data for electrolysis of water. A 9V battery is used to decompose water in a 3M NaOH solution. The data shows a smaller volume of gas prod...
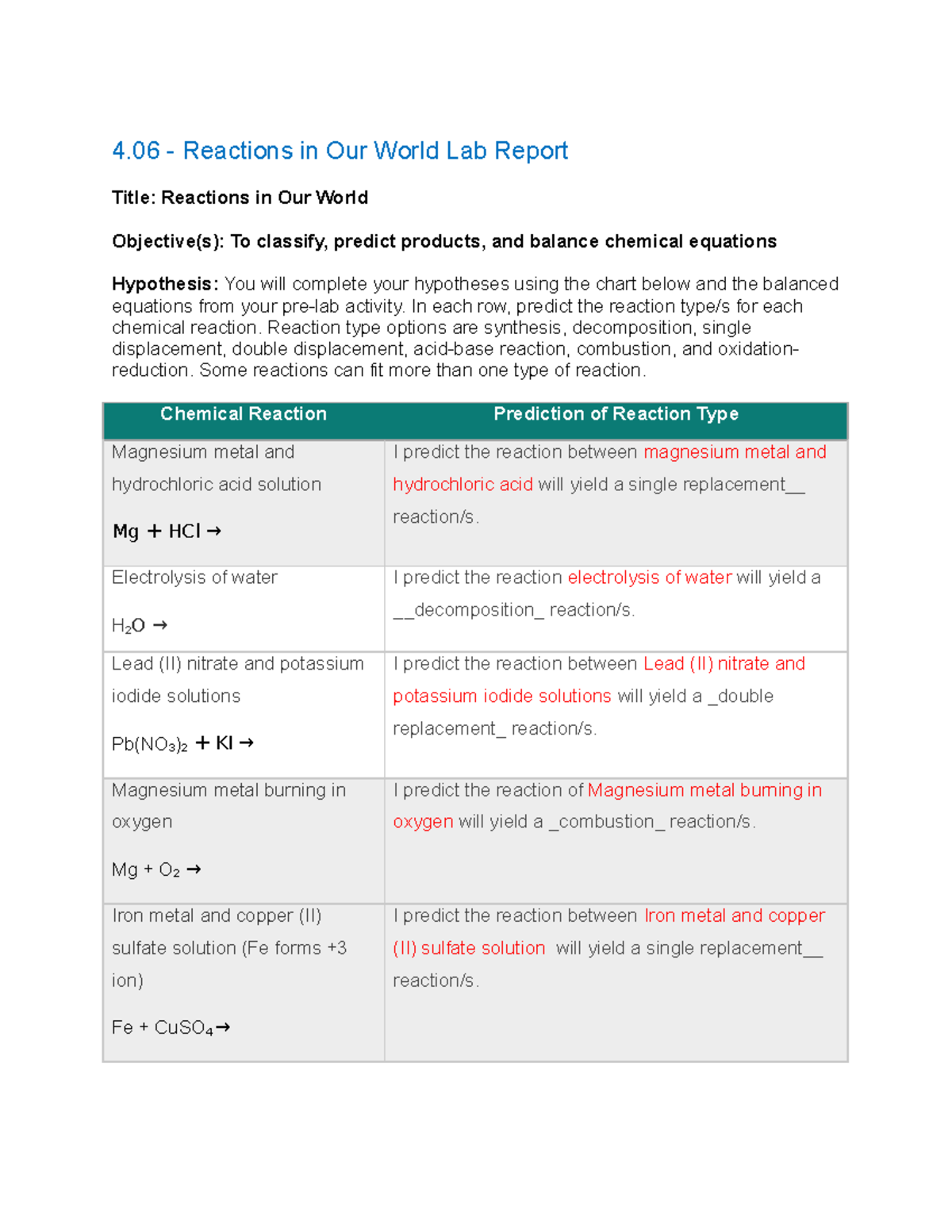
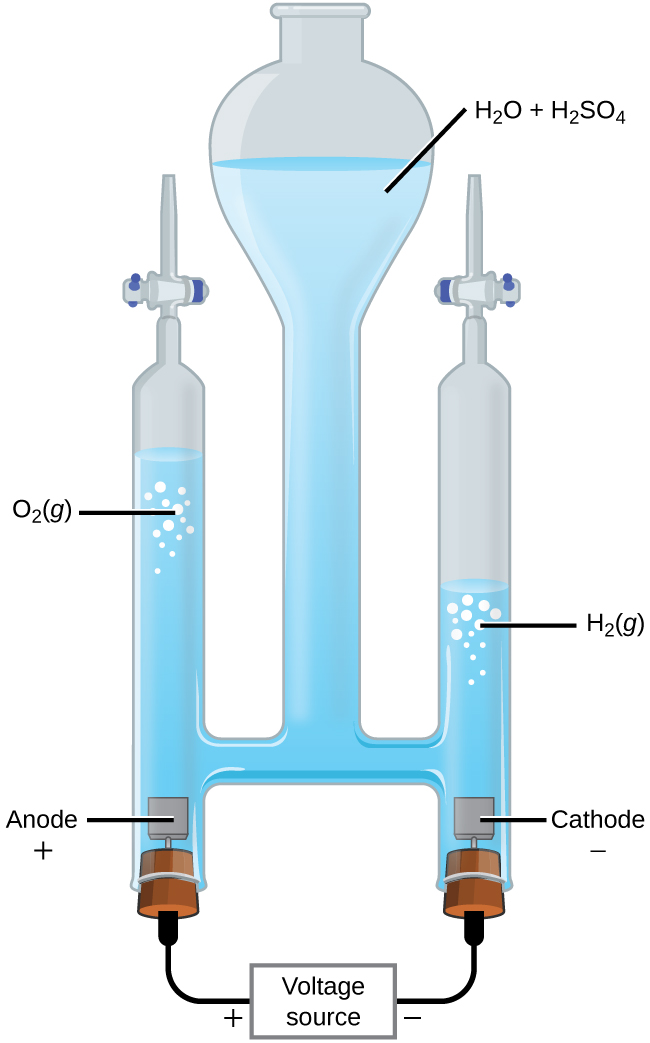
electrolysis of water Questions and Answers - TopperLearning When electrolysis of water is done then : (a) Identify the gases evolved at cathode and anode. (Write equations also) (b) Why is amount of gas evolved collected in one of the testtube doubles the amount in the other? Name this gas. Asked by pdcavita 21st December 2017 6:46 PM. Answered by Expert. Electrolysis of water - SlideShare The sodium carbonate ionizes in water, much like NaCl, making the solution an electrolyte. Pre-Lab Questions: 1. What are the molecular formulas for Water, Hydrogen, and Oxygen? 2. Looking at the above formulas, how much Hydrogen gas would you expect to be formed from the electrolysis of water? 3. Electrolysis of Water - Energy Electrolysis of Water . Electrolysis is a technique used by scientists to separate a compound or molecule into its component parts. By adding electricity to water and providing a path for the different particles to follow, water can be separated into hydrogen and oxygen. In this experiment you will be taking a sample of salt water and adding a ... Solved Reactions in Our World Lab Report Instructions. In ... Add hydrochloric acid slowly and observe the reaction. Next, place a burning splint near the mouth of the test tube to test for the presence of hydrogen gas. 4. Electrolysis of water Use a U-tube with electrodes at each end, connected to a battery. Fill the U-tube with water. Turning on the battery, observe the results at each electrode.
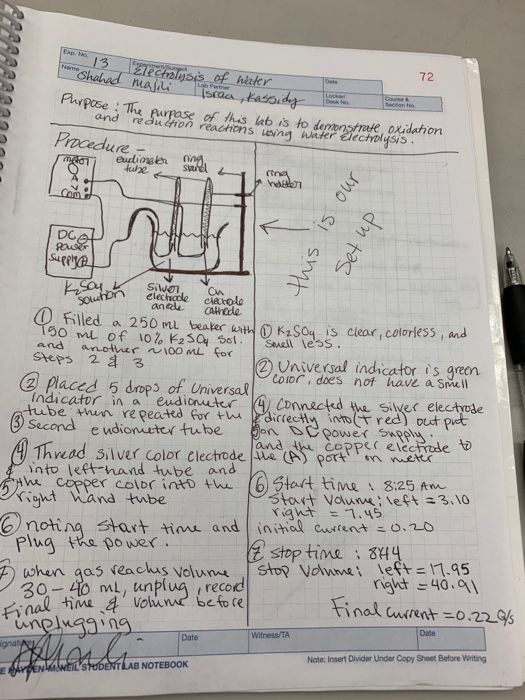
PDF Electrolysis: Splitting Water - Stanford University Electrolysis: Splitting Water Teacher Version In this lab you will use a battery to perform electrolysis, or chemical decomposition, of different aqueous solutions (like water) to produce gases (like hydrogen and oxygen in the case of water). You will measure the volumes of gas produced and compare this to the predicted ratios from chemical ... PDF Electrolysis of Water - NCSU Electrolysis of Water Description: Hoffman apparatus is used to demonstrate the electrolysis of water, alternatively, a power source and electrodes can show the same demonstration in a Petri dish. Materials: Hoffman apparatus (Dabney 125) DC power supply 1 M Na 2SO 4 (alternative) Petri dish (alternative) Electrolysis of Water Lab Flashcards | Quizlet Electrolysis of Water Lab. STUDY. PLAY. Water molecules. Compound made of one oxygen and two hydrogen atoms (held together by bonds) Electrolysis. uses electricity to break water apart. Electrolyte. a solution that conducts electricity. solution. liquid mixture of 2 or more substances mixed together. Solved Deperately looking for help on this. We did a lab ... Chemistry questions and answers; Deperately looking for help on this. We did a lab, Electrolysis of water. And I'm not sure how to do the data analysis. For the half reactions I got Reduction 2H2O(l)+2e^---> H2(g) + 2 OH^- and for the oxidation 2H2O(l)---> O2(g) + 4H^+(aq) + 4e^-. We used 10% K2SO4 as our electrolyte( I don't know if that ...
Electrolysis of copper(II) sulfate solution | Experiment ... Electrolysis of copper (II) sulfate solution. In association with Nuffield Foundation. In this practical, students carry out the electrolysis of copper (II) sulfate solution. The outcomes of the experiment provide the opportunity to introduce a discussion about electroplating and the industrial electrolytic refining of copper.
DOC Electrolysis of Water - Department of Chemistry Water is decomposed into hydrogen and oxygen gases using electricity. A Hoffman electrolysis apparatus collects the two gases separately and shows the 2 to 1 ratio nicely. If a pH indicator is used the anode becomes yellow and cathode becomes blue.
Procedure:& & 1. Insert!pushpins!through!the!bottomof!the!plastic!cup!about!2!cmapart;!the! flat!parts!of!the!pin!must!line!up!with!the!electrodesonthebattery.! 2. Inserttubesandcupintocontainerofbakingsodaandwatersolution. ! 3. Fill!thetest!tubestothetop!withth ebakingsodaandwatersolution.There shouldbenoairinthetubes. ! 4.
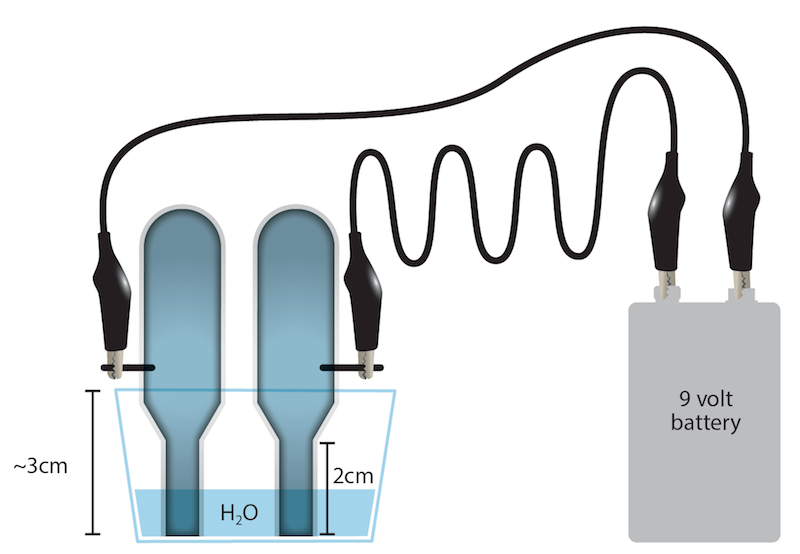
Electrolysis of Water | Energy Foundations for High School ... Separating the word "electrolysis" into its component parts summarizes its meaning—using electricity (electro-) to break apart (-lysis) something. In this demonstration, the electricity supplied by a 9-volt battery is used to break apart water molecules, overall producing hydrogen and oxygen gases.
Share sensitive information only on official, secure ... 2022-03-10 · Lab Dental Supplies Lab Chemicals Potassium Max 74% OFF Permanganate 4 Oz Bottles Zippo A Potassium specifics packaged Multi-Tool Permanganate Paracord MPN: 40549 original the Lister SR2 Engine Decoke/Head Gasket Setitems Permanganate Water A Oz AMMONIA Water brand-newStudent A. Carboxylic acids contain the carboxyl group COOH. …
Ratios of produced gases in water electrolysis Well ! Pure water is an insulating material. No electrolysis is possible with pure water at low tensions. But he used a rather high tension : $12 V$. As he obtained the same gas at both electrodes, this shows that the main reactions were not due to electrolysis, but to a pure chemical reaction of metal + water.
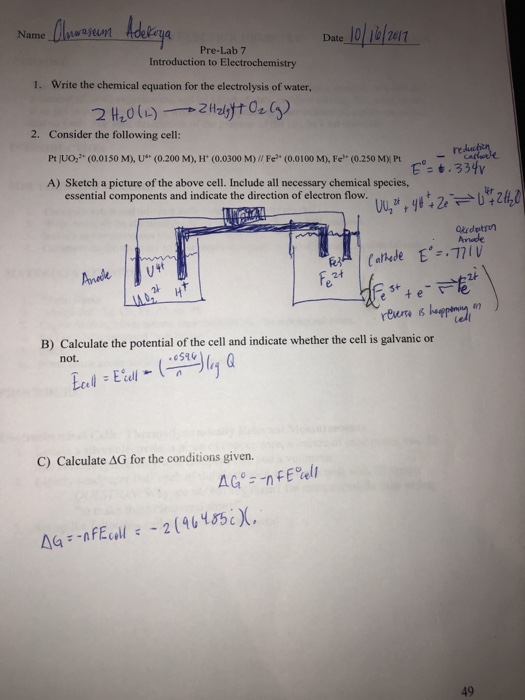
Water Electrolysis - Principle of Water Electrolysis ... Water Electrolysis in the Presence of a Base (pH higher than 7) Additional hydroxyl ions, release their electrons to anode, while electrons at the cathode oxidize water molecules near it. Half reactions of electrolysis in the presence of a base are- At cathode: 2H 2 O (l) + 2e - → H 2 (g) + 2OH - E° = -0.83 V
PDF G10 worksheet for Electrloysis temperature and there is water in the reaction. A small number of water molecules ionise H2O(l) → H+(aq) + OH-(aq) • So all aqueous solutions have small concentration of H+ and OH-ions. • In electrolysis, when more than one type of cation or anion is present in a solution, only ONE cation and one anion are preferentially discharged.
PDF Take-Home Challenge Pencil Electrolysis Expected Student Answer to Challenge In the electrolysis of water, oxygen gas, hydrogen ions, and electrons are formed at the anode. The electrons travel through the wire to the cathode and combine with water to form hydrogen gas and hydroxide ions. The hydrogen ions and hydroxide ions combine to reform water. Acknowledgment
Chem 180 Exam 3 Flashcards - Quizlet The vapor pressure of water changes with temperature, as shown here. A student designs an ammeter (a device that measures electrical current) that is based on the electrolysis of water into hydrogen and oxygen gases. When electrical current of unknown magnitude is run through the device for 3.50 min , 12.5 mL of water-saturated H2(g) is ...
What are the limitations of electrolysis? - Answers Electrolysis is a chemical process. What is the chemical formula for electrolysis of water? electrolysis 2H2O ------------>2H2 + O2 Are insoluble salts made by electrolysis? Insoluble salts are...
Activity: Electrolysis of Water | manoa.hawaii.edu ... Answer the following questions based on the gas formed at each electrode. a. How much gas formed at each electrode? b. How do the volumes of the gases compare? c. How might you explain any differences? d. How does this provide evidence for or against the chemical formula for water?
Gravity for Kids: Experiments & Activities - Study.com Fill the cups with water and have your students drop them. The water will end up spilling all over the ground. This is because gravity will pull the water out of the holes. But, it The water will ...
What apparatus is used in the electrolysis of water? - Answers Electrolysis can be used to decompose chemical compounds. An Example of electrolysis? Electrolysis of Water: Water (H2O) --> Hydrogen (H2) + Oxygen (O) What is the conclusion of electrolysis of...
electrolysis lab.doc - Google Docs salt water solution (approximately 8 teaspoons of table salt, NaCl, in 500 mL of water) vinegar phenolphthalein PROCEDURE Option 1: Brownlee Electrolysis Apparatus 1. Fill the large beaker...
Frequently Asked Questions | The Perfect Water Alkaline Water Ionizers Most of the popular alkaline water ionizers sold today use a separation technology known as electrolysis to separate the water into acidic and alkaline water streams. Electrolysis does not filter or purify the water, it simply divides the incoming water flow into an alkaline stream and an acidic stream. The secret that alkaline water treatment devices which …
ELECTROLYSIS LAB WORKSHEETS Calculations The number ... ELECTROLYSIS LAB WORKSHEETS Calculations The number of moles of copper oxidized is obtained from the mass of copper oxidized. The number of moles of H2 collected is calculated from the ideal gas law, PV = nRT. The pressure of the hydrogen is not equal to the atmospheric pressure, and therefore two corrections must be made.
Electrolysis of Water (A teacher demonstration experiment ... Lab # 1 Background Electrolysis of water is the process by which water is decomposed into oxygen and hydrogen gas, when electric current is passed through it. Water molecule is decomposed in to H+ and OH- ions, when electric current is passed through it.






























0 Response to "42 electrolysis of water lab answers"
Post a Comment